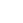- Synonyms
- 14 kDa Selenium-binding Protein, Fatty Acid-binding Protein 1, L-FABP
- Source
- Escherichia coli.
- Molecular Weight
- Approximately 14.2 kDa, a single non-glycosylated polypeptide chain containing 127 amino acids.
- AA Sequence
- MNFSGKYQLQ SQENFEPFMK AIGLPEDLIQ KGKDIKGVSE IVHEGKKIKL TITYGPKVVR NEFTLGEECE LETMTGEKVK AVVKLEGDNK MVTTFKGIKS VTELNGDTIT NTMTLGDIVY KRVSKRI
- Purity
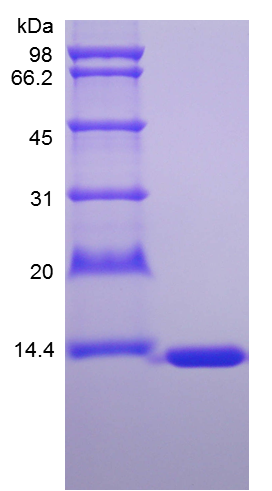
- > 95 % by SDS-PAGE and HPLC analyses.
- Biological Activity
- Fully biologically active when compared to standard. The binding affinity of rMuFABP1 for the synthetic ligand cis-parinaric acid has been measured by fluorescence titration. Half maximal fluorescence of 2.5 μM rMuFABP1 is achieved with approximately 5 μM cis-paranaric acid.
- Physical Appearance
- Sterile Filtered White lyophilized (freeze-dried) powder.
- Formulation
- Lyophilized from a 0.2 µm filtered concentrated solution in PBS, pH 7.4, 2 % trehalose.
- Endotoxin
- Less than 1 EU/µg of rMuFABP1 as determined by LAL method.
- Reconstitution
- We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Reconstitute in sterile distilled water or aqueous buffer containing 0.1 % BSA to a concentration of 0.1-1.0 mg/mL. Stock solutions should be apportioned into working aliquots and stored at ≤ -20 °C. Further dilutions should be made in appropriate buffered solutions.
- Stability & Storage
- Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 3 months, -20 to -70 °C under sterile conditions after reconstitution.
- Usage
- This material is offered by Shanghai PrimeGene Bio-Tech for research, laboratory or further evaluation purposes. NOT FOR HUMAN USE.
- SDS-PAGE
- Reference
- 1. Chmurzynska A. 2006. J Appl Genet. 47:39-48.
2. Weisiger RA. 2002. Mol Cell Biochem. 239:35-43.
3. Chen SH, Van Tuinen P, Ledbetter DH, et al. 1986. Somat Cell Mol Genet. 12:303-6.
4. Weickert MO, Loeffelholz CV, Roden M, et al. 2007. Am J Physiol Endocrinol Metab. 293:E1078-84.
5. Bassuk JA, Tsichlis PN, Sorof S. 1987. Proc Natl Acad Sci U S A. 84:7547-51.
6. He Y, Yang X, Wang H, et al. 2007. Biochemistry. 46:12543-56.
- Background
- The fatty-acid-binding proteins (FABPs) are a family of carrier proteins for fatty acids and other lipophilic substances such as eicosanoids and retinoids. These proteins are thought to facilitate the transfer of fatty acids between extra- and intracellular membranes. Fatty acid-binding protein 1 (FABP1) encoded by the FABP1 gene, also known as liver-type fatty acid-binding protein (L-FABP), is a member of FABP family and it is a small, highly conserved, cytoplasmic proteins. In addition, FABP1 binds free fatty acids and their coenzyme A derivatives, bilirubin, and some other small molecules in the cytoplasm. Furthermore, it may be involved in intracellular lipid transport. Through amino acid sequence comparison, murine FABP1 shares 84 % and 94 % a.a. sequence identity with human and rat FABP1.








 COA Application
COA Application