- Source
- Escherichia coli.
- Molecular Weight
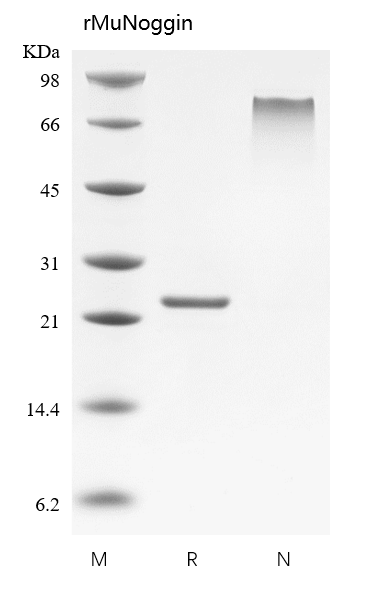
- Approximately 46.4 kDa, a disulfide-linked homodimer consisting of two 206 amino acid polypeptide chains.
- AA Sequence
- MQHYLHIRPA PSDNLPLVDL IEHPDPIFDP KEKDLNETLL RSLLGGHYDP GFMATSPPED RPGGGGGPAG GAEDLAELDQ LLRQRPSGAM PSEIKGLEFS EGLAQGKKQR LSKKLRRKLQ MWLWSQTFCP VLYAWNDLGS RFWPRYVKVG SCFSKRSCSV PEGMVCKPSK SVHLTVLRWR CQRRGGQRCG WIPIQYPIIS ECKCSC
- Purity
- > 95 % by SDS-PAGE and HPLC analyses.
- Biological Activity
- Fully biologically active when compared to standard. The ED50 as determined by inhibiting BMP-4-induced alkaline phosphatase production of murine ATDC5 cells is less than 2 ng/ml, corresponding to a specific activity of > 5.0 × 105 IU/mg in the presence of 5 ng/ml BMP-4.
- Physical Appearance
- Sterile Filtered White lyophilized (freeze-dried) powder.
- Formulation
- Lyophilized from a 0.2 μm filtered concentrated solution in 30 % acetonitrile, 0.1 % TFA.
- Endotoxin
- Less than 1 EU/μg of rMuNoggin as determined by LAL method.
- Reconstitution
- We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Reconstitute in 10 mM HAc to a concentration less than 0.25 mg/mL. Stock solutions should be apportioned into working aliquots and stored at ≤ -20 °C. Further dilutions should be made in appropriate buffered solutions.
- Stability & Storage
- Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 3 months, -20 to -70 °C under sterile conditions after reconstitution.
- Usage
- This material is offered by Shanghai PrimeGene Bio-Tech for research, laboratory or further evaluation purposes. NOT FOR HUMAN USE.
- Reference
- 1. Davis SWandCamper SA. 2007. Dev Biol, 305: 145-60.
2. Zhu W, Kim J, Cheng C, et al. 2006. Bone, 39: 61-71.
3. Oxley CD, Rashid R, Goudie DR, et al. 2008. Horm Res, 69: 221-6.
4. Cooper GM, Usas A, Olshanski A, et al. 2009. Plast Reconstr Surg, 123: 94S-103S.
5. Bayramov AV, Eroshkin FM, Martynova NY, et al. 2011. Development, 138: 5345-56.
- Background
- Noggin encoded by the NOG gene, was first isolated from Xenopus, having the function of inducing secondary axis formation in frog embryos. It inhibits TGF-β family ligands and preventing them from binding to their corresponding receptors. Noggin was originally found as a BMP-4 antagonist, and then has been shown to modulate the activities of other BMPs (BMP-2, 7, 13 and 14). Additionally, it has pleiotropic effect, both in early development and later stages. The results of the mouse knockout of noggin suggest that it is involved in numerous developmental processes, such as neural tube fusion and joint formation. In recent report, proximal symphalangism (SYM1) and multiple synostoses syndrome (SYNS1) have relation with the mutant of evolutionarily conserved amino acid residues of Noggin. Mature mouse Noggin shares 99 % and 83 % a.a. sequence identity with human and Xenopus Noggin, respectively.








 COA Application
COA Application