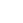- Source
- Insect Cell
- Molecular Weight
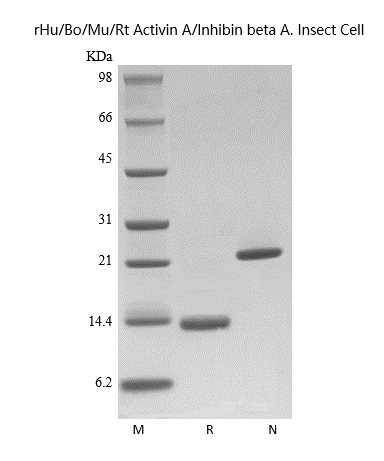
- Approximately 26.0 kDa, a homodimeric protein consisting of two 116 amino acid polypeptide chains.
- AA Sequence
- GLECDGKVNI CCKKQFFVSF KDIGWNDWII APSGYHANYC EGECPSHIAG TSGSSLSFHS TVINHYRMRG HSPFANLKSC CVPTKLRPMS MLYYDDGQNI IKKDIQNMIV EECGCS
- Purity
- > 95 % by SDS-PAGE analyses.
- Biological Activity
- Test in processing.
- Physical Appearance
- Sterile Filtered White lyophilized (freeze-dried) powder.
- Formulation
- Lyophilized from a 0.2 μm filtered concentrated solution in 20 mM Tris-HCl, pH 8.0, 300 mM NaCl, with 5 % Trehalose, 0.02 % Tween-20.
- Endotoxin
- Less than 0.1 EU/μg of rHu/Bo/Mu/Rt Activin A/Inhibin beta A, Insect Cell as determined by LAL method.
- Reconstitution
- We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Reconstitute in sterile 10mM HCl to a concentration of 0.1-0.3 mg/mL. Stock solutions should be apportioned into working aliquots and stored at ≤ -20 ℃. Further dilutions should be made in appropriate buffered solutions.
- Shipping
- The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below.
- Stability & Storage
- Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 3 months, -20 to -70 °C under sterile conditions after reconstitution.
- Usage
- This material is offered by Shanghai PrimeGene Bio-Tech for research, laboratory or further evaluation purposes. NOT FOR HUMAN USE.
- SDS-PAGE
- Background
- Activin and Inhibin are members of the TGF-beta superfamily of cytokines and are involved in a wide range of biological processes including tissue morphogenesis and repair, fibrosis, inflammation, neural development, hematopoiesis, reproductive system function, and carcinogenesis. Activin and Inhibin are produced as precursor proteins. Their amino terminal propeptides are proteolytically cleaved and facilitate formation of disulfide-linked dimers of the bioactive proteins. Activins are nonglycosylated homodimers or heterodimers of various beta subunits (beta A, beta B, beta C, and beta E in mammals), while Inhibins are heterodimers of a unique alpha subunit and one of the beta subunits. Activin A is a widely expressed homodimer of two beta A chains. The beta A subunit can also heterodimerize with a beta B or beta C subunit to form Activin AB and Activin AC, respectively. The mature human beta A chain shares 100% amino acid sequence identity with bovine, feline, mouse, porcine, and rat beta A. Activin A exerts its biological activities by binding to the type 2 serine/threonine kinase Activin RIIA which then noncovalently associates with the type 1 serine/threonine kinase Activin RIB/ALK-4. Signaling through this receptor complex leads to Smad activation and regulation of activin-responsive gene transcription. The bioactivity of Activin A is regulated by a variety of mechanisms. BAMBI, Betaglycan, and Cripto are cell‑associated molecules that function as decoy receptors or limit the ability of Activin A to induce receptor complex assembly. The intracellular formation of Activin A can be prevented by the incorporation of the beta A subunit into Activin AC or Inhibin A. And the bioavailability of Activin A is restricted by its incorporation into inactive complexes with alpha 2-Macroglobulin, Follistatin, and FLRG.








 COA Application
COA Application